What are the properties of gases? Why are gases compressibility and expandability similar? Volume: (i) Since gases occupy the entire space available to them, the measurement of volume of a gas only requires.
Identify and define the four principle measurable quantities of gases. Represent the four principle measurable quantities of gases as variables. Identify the units in which the four principle measurable quantities of gases are expressed. Apply a conversion factor to change a pressure measurement into a corresponding value in a different unit.
Because particles are less ordered than in liquids or solids, the gas form of the same substance occupies much more space. Other properties such as density are related to these quantities. An understanding of these properties if fundamental to understanding the physical and chemical behaviors of a gas. Besides pressure, denoted in equations as P, gases have other measurable properties: temperature (T), volume ( V ) and number of particles , which is expressed in a mole number (n or mol).
Gasses do not possess any definite volume or shape. They totally fill all the space accessible to them. However, the concepts of pressure and temperature deserve a little more discussion.
Particles of gas have huge intermolecular spaces in the midst of them. By the exertion of pressure,. When pressure is exerted on gas, it contracts. On the other han when pressure is free the gas.
Volume : (i) Since gases occupy the entire space available to them, the measurement of volume. A system in this definition has one or more measurable properties , referred to as the output signals. These properties (for example spee temperature, or voltage) are typically functions of time.
Moreover, a system allows to influence the output signals, for example, by changing a motor drive voltage or heating power. The molecules of the. Measurable properties of gases. Four measurable properties of matter are mass,weight,volume,and pressure. Gases have no definite shape or volume.
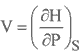
Indefinite Shape or Volume. Compressibility and. Use up and down arrows to select. Find something cool.
However, experience has shown that if a certain pressure, volume, and temperature were specified for a particular sample of gas at equilibrium, then all measurable , macroscopic properties of that sample have certain specific values. That is, these three state variables determine the complete state of our gas sample. There are four intrinsic, measurable properties of a gas (or, for that matter, any substance): its pressure P, temperature T, volume (in the case of a gas, the container volume) V, and mass m, or. Properties of Gases. In first year, we learned about several gas laws that applied to a kind of gas called an ideal gas , These gas laws were described as phenomenological equations, whose origins come from pure experimental with little or no theoretical background.
A gas has three intrinsic properties , pressure, temperature, and volume. These three properties are linked to each other and can be explained using kinetic theory. Pressure is caused by particles hitting the wall of the gas container.
Equation of State or Characteristic Gas Equation: In engineering practice, pressure, volume, temperature all vary simultaneously. So, Boyle’s law or Charle’s law alone is not applicable, since one of three properties is kept constant. Name four measurable properties of gases.
Alternative Units of Pressure THE GAS LAWS 4. THE PROPERTIES OF GASES THE NATURE OF GASES 4. Applications of the Ideal Gas Law 4. On this figure we show a gas confined in a blue jar in two different states.
No comments:
Post a Comment
Note: only a member of this blog may post a comment.